What Year Did The Food And Drug Administration (fda) Approved Depo-provera
In 1983 the FDA refused approval of Depo-Provera a third time. It is a white to off-white odorless crystalline powder stable in air.
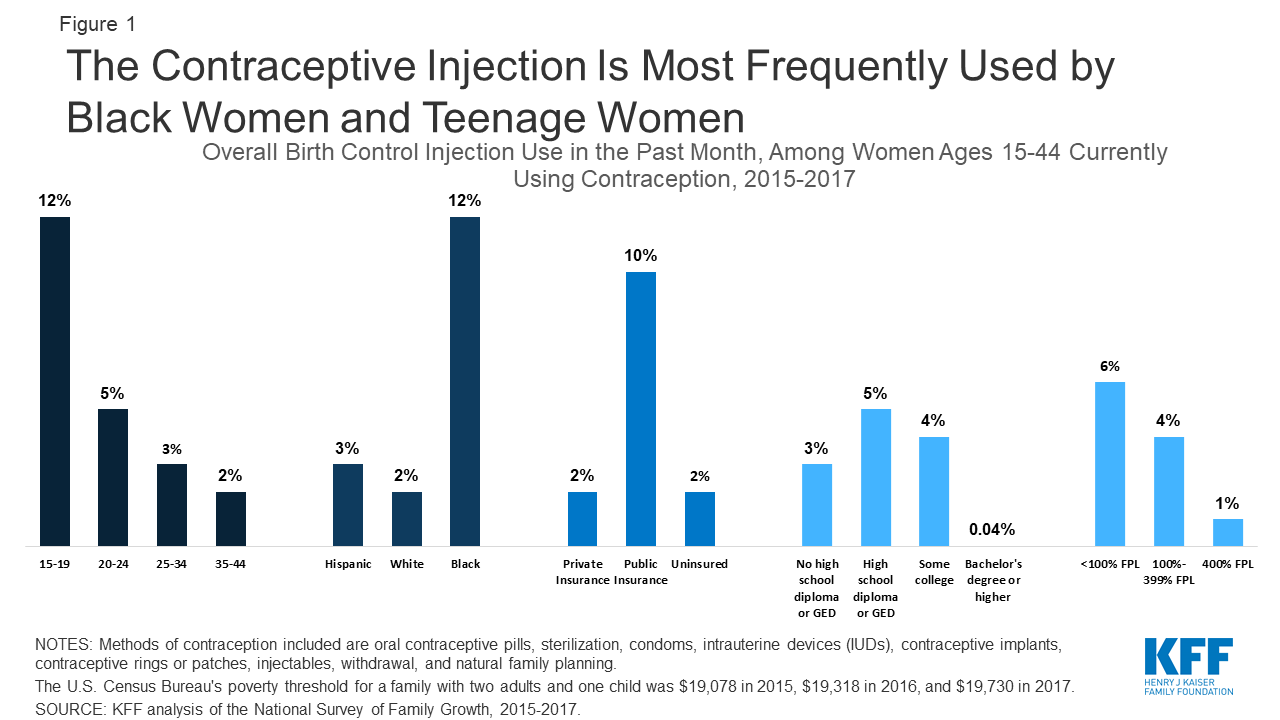
Dmpa Contraceptive Injection Use And Coverage Kff
Black Box Warning on the risk ofosteoporosis.
What year did the food and drug administration (fda) approved depo-provera. Clinical trials began in 1963. Although the verdict will be based on considerations for American women only. Should any of these occur or be suspected the drug should be discontinued immediately.
The final decision is expected to have major economic and social implications. Eventually in 1992 the FDA approved the Depo-Provera injection for use in the United States over the objections of pro-life groups and even several pro-abortion womens organizations including the National Womens Health Network and. Upjohn first applied to the Food and Drug Administration FDA for permis-sion to market Depo-Provera as a con-traceptive in 1967.
Depo-subQ provera 104 is a new formulation of Pfizers Depo-Provera which the FDA approved for use as a contraceptive in December 2004. In October 1992 the US Food and Drug Administration FDA approved Depo-Provera for contraceptive use thus increasing the number of available contraceptives to women. DEPO-PROVERA Sterile Aqueous Suspension contains medroxyprogesterone acetate which is a derivative of progesterone and is active by the parenteral and oral routes of administration.
Depo-Provera had been approved as safe for medical uses other than birth control around 1960. Depo-Provera depo-medroxyprogesterone acetate is an injectable form of birth control approved by the US. Plagued the Food and Drug Administration FDA in U.
The panel has b een asked to recommend whether Depo-Provera should be approved for use as an injectable contraceptive. Then in 2004 the agency revised the drugs labeling to include a boxed ie. Ing that Depo-Provera depot medroxy-progesterone acetate administered by injection was a long-lasting and effective contraceptive.
100MGML Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons. FDA first approved the drug in 1959 to treat amenorrhea5 irregular uterine bleeding and threatened and habitual abortion. Then in September 1974 the FDA announced in the Federal Register its intention to approve use of Depo-Provera as a contraceptive.
FDA Approved Drugs and User Comments. Dosage FormRoute Marketing Status. This article will analyze the FDAs Depo-Provera approval and label revision process.
I did get pregnant last year after not having the shot for about six months. Food and Drug Administration FDA in 1992. Twelve years and many.
The FDA approved Depo-Provera an injectable contraceptive in 1992 on the condition that its manufacturer conduct a post-approval study on the risk ofosteoporosis. Depo-Provera was developed in the 1960s and already is sold in more than 90 countries--including the United Kingdom Germany France New. Prior to 1961 new drugs had to demonstrate only that they were safe before receiving FDA approval for marketing.
Prior to 1961 new drugs had to demonstrate only that they were safe before receiving FDA approval for marketing. Depo-Provera Contraceptive Injection should not be used as a long-term birth control method ie longer than 2 years unless other birth control methods are considered inadequate. See Warnings and Precautions 51.
The injection also known as the Depo shot or DMPA is given four times a year and helps women of childbearing age avoid. The loss of bone mineral density. The following year it was approved to treat endometriosis6 Later FDA withdrew its approval for use of the drug in preventing miscarriage and endome triosis7 In 1972 FDA also approved Depo-Provera as adjunctive therapy.
After nearly 20 years of controversy the Food and Drug Administration yesterday approved Upjohn Cos Depo Provera an injectable contraceptive that protects women against pregnancy for up to. Food and Drug Administration FDA in 1992 and the drug is currently manufactured by pharmaceutical giant Pfizer. 1 INDICATIONS AND USAGE Depo-Provera CI is indicated only for the prevention of pregnancy.
Yet USAID has distributed it through its family planning programs in. The data described below reflect exposure to depo-subQ provera 104 in five clinical trials involving 2325 women including 2043 women who received treatment for contraception 1780 treated up to 1 year and 263 treated for up to 2 years and 282 women for endometriosis for up to 6. It was first introduced in the United States in 1959 for management of menstruation and was approved for contraceptive use by the US.
Eventually in 1992 the FDA approved the birth control shot DepoProvera injection for use in the United States over the objections of pro-life groups and even several pro-abortion womens organizations including the National Womens Health Network and the National Black Womens Health Project. In February 1973 an OB-GYN Expert Advisory Committee to the FDA recommended limited approval of Depo-Provera as a contraceptive-only with patient consent and information for patients describing the drugs hazards. Depo-Provera had been approved as safe for medical uses other than birth control around 1960.

Fda Approves 1st Birth Control App Vaginal Contraception Ring Cnn

Covid U S States Face Steep Decline In J J Vaccine

Us Could Authorise Pfizer Covid 19 Shot For Kids Aged 5 To 11 In October World News Hindustan Times

Health Facts Depo Provera And Bone Mineral Density National Women S Health Network

Depo Provera Medroxyprogesterone Uses Dosage Side Effects Interactions Warning

Dmpa Contraceptive Injection Use And Coverage Kff

Health Facts Depo Provera And Bone Mineral Density National Women S Health Network
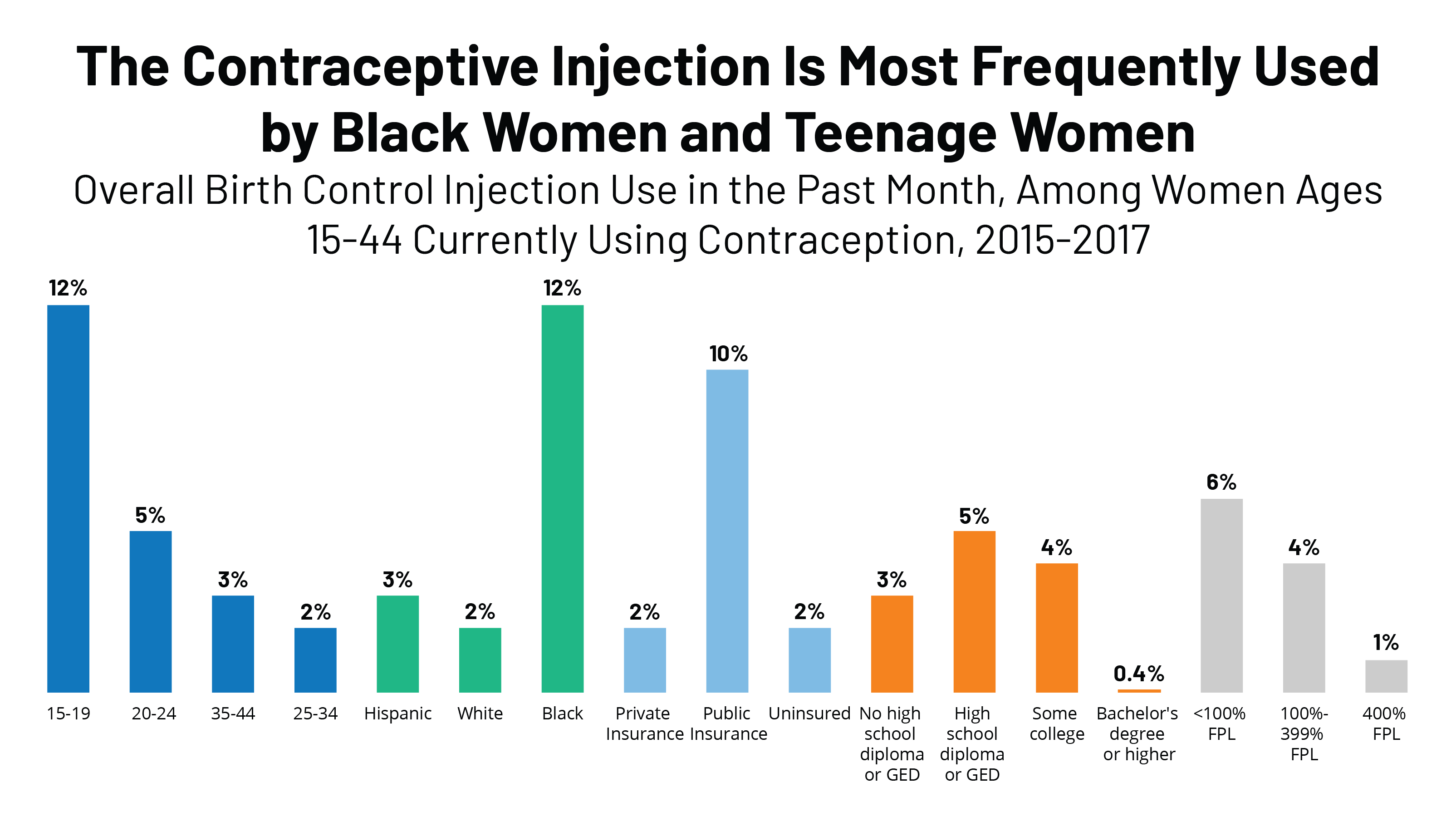
Dmpa Contraceptive Injection Use And Coverage Kff

Food And Drug Administration Cbs New York
Depo Shot 101 Everything You Need To Know About Depo Provera
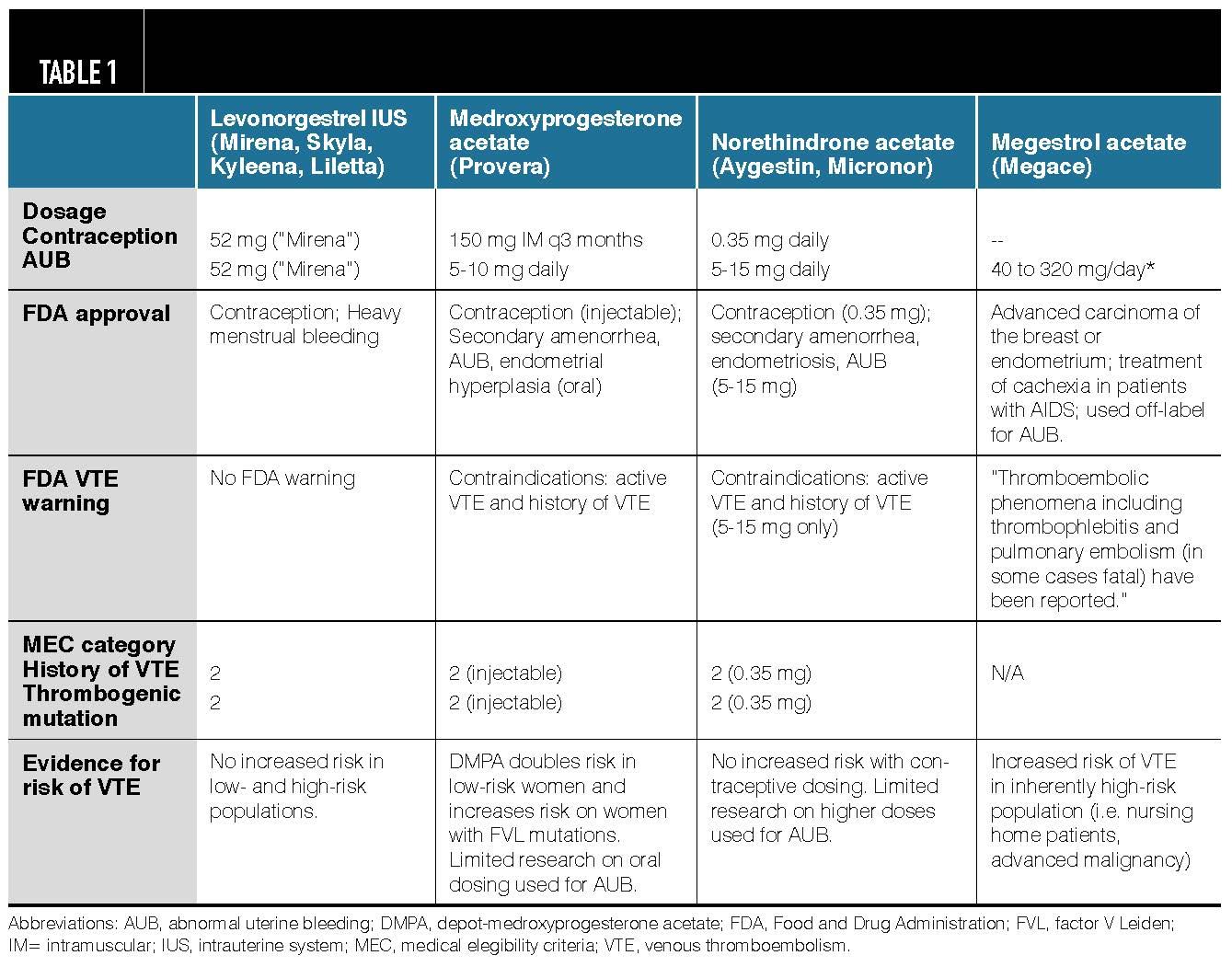
Progestins For Aub In Women At Risk Of Vte
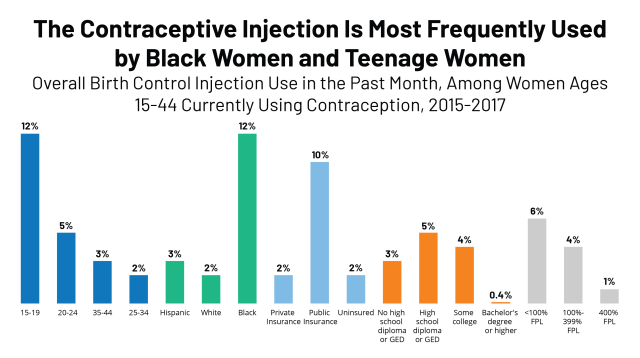
Dmpa Contraceptive Injection Use And Coverage Kff

U S Fda May Not Review New Covid 19 Vaccine Eua Requests During Pandemic
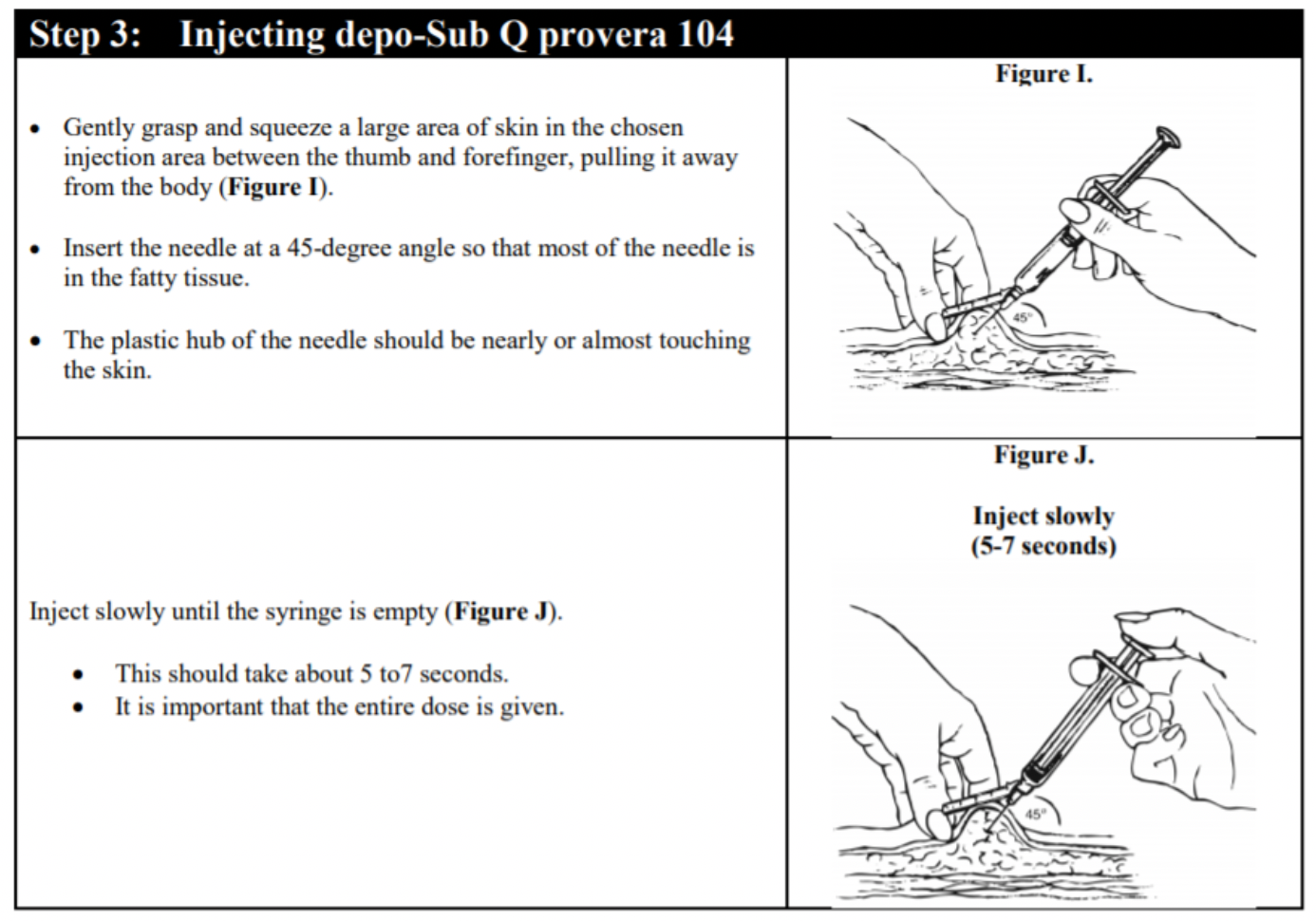
Post a Comment for "What Year Did The Food And Drug Administration (fda) Approved Depo-provera"