Food Drug And Cosmetic Act India
One Naushad Khan made an online complaint on 2222018. Under the Act arrest a person in connection with an offence under Chapter IV of the Act.

Indian Drug Manufacturer Sentenced To Pay 50 Million In Fines Deccan Herald
The Commissioner Food Protection and Drugs directed enquiry and the Drug Inspector Mau UP.

Food drug and cosmetic act india. Kala Nagar Bandra East. 341 2nd Floor Bandra. Presently the governing legislation in case of cosmetics in India is the Drugs Cosmetics Act 1940 which defines a cosmetic as any article intended to be rubbed poured sprinkled or sprayed on or introduced into or otherwise applied to human body or any part thereof for cleansing beautifying promoting attractiveness or altering the appearance and.
Terms of section 103 of the Government of India Act 1935 26 Geo. 371a4 Gives FDA the authority to request records in advance of an inspection or in lieu of inspection. 15-9-1987 or any recognised consumer.
Drugs and Cosmetics Act 1940 hereinafter referred to as the Act and Rules 1945 hereinafter referred to as the Rules were passed with the aim to ensure that only qualified personnel are engaged in manufacturing importing distribution and sale of drugs and cosmetics confirming safe and effective drugs and cosmetics are being sold. 6 of 1963 s. The Federal Food Drug and Cosmetic Act and subsequent amending statutes are codified into Title 21 Chapter 9 of the United States Code.
The Food and Drug Administration FDA of Maharashtra is a trusted agency in the state to enforce the Food Drug and Cosmetic Act fairly upholds safety standards and protects the consumers. India Food Drugs Healthcare Life Sciences Food and Drugs Law Media Telecoms IT. Central Drug Standards Control Organization is the Regulatory expert for the endorsement of New Drug Import Registration of Drugs Medical Devices and Cosmetics.
Drugs and Cosmetics Act 2008-GUIDELINES UNDER NEW PENAL PROVISION. Kurla Complex Opposite Reserve Bank Of India. Under Drug Cosmetics Act Rules Licenses Registration Food Safety and Drugs Administration Government of Uttar Pradesh India.
Get details on the Drugs and Cosmetics Amendment Act 2008 which includes amendments in the Drugs and Cosmetics Act 1940. The primary objective of the Act is to ensure that the drugs and cosmetics sold in India are safe effective. The Act prohibits the manufacture import and sale of substandard spurious and adulterated drugs.
The act provide for. The act regulates the import of drugs in India so that no substandard or spurious drug will enter into our country. Users can access information about the Act its objectives short title and commencement.
Drugs and Cosmetics Act 2008-THE DRUGS COSMETICS AMENDMENT ACT 2008. Under Rule 106 of the Drugs and Cosmetics Act 1940 a drug cannot make claims to treat or prevent any of the diseases or reform the conditions listed. Drugs and Cosmetics Amendment Act 2008.
The Drugs and Cosmetics Act and Rules are the administering directions in India. Food and Drug Administration. The Parliament of India formed an Act which regulates the import manufacture and distribution of drugs in India.
Dated ththe 20 July 1968 Gazette of India Pt. The act prohibits the manufacture of substandard or spurious drug in the country. Other than food intended to affect the structure or any function of the human.
Any person Note. It is a trusted agency to enforce the Food Safety Standards Act 2006 Drug and Cosmetics Act fairly upholds safety standards and protects consumers. Preparedness prior to a crisis FDC Act 704a4 was in place before COVID-19 Under section 704a4 of the Federal Food Drug and Cosmetic Act 21 USC.
Purchaser of drug or cosmetic enabled to obtain test or analysis. Along with two others conducted an inspection at the Sharda Narayan Clinic. Food and Drug Administration Maharashtra State is the State prime organization of consumer protection.
The Act is extended to Dadra and Nagar Haveli by Reg. The Drugs and Cosmetics Act 1940 as amended from time to time regulates the import manufacture sale and distribution of drugs and cosmetics in the country. Drugs And Cosmetics Act.
Drugs and Cosmetics Act 1940 and Rules 1945 As amended up to the 31st December 2016. It has dedicated professionals working to protect promote and enhance the health of people. An Act further to amend the Indian Penal Code the Code of Criminal Procedure 1973 the Drugs and Cosmetics Act 1940 and the Prevention of Food Adulteration Act 1954 in their application to Uttar Pradesh with a view to taking more effective action against adulteration of food drugs and cosmetics and for providing deterrent punishments for.
DCA Drugs and Cosmetics Act 1940 DCGI Drugs Controller General of India DGHS Director-General of Health Services DI Drug Inspector DTAB Drugs Technical Advisory Board DCC Drugs Consultative Committee EMA European Medicines Agency EUDRA European Union Drug Regulatory Authorities FSSAI Food Safety and Standards Authority of India. THE DRUGS AND COSMETICS ACT AND RULES THE DRUGS AND COSMETICS ACT 1940. Guidelines for drug food and cosmetics are also available.
Details regarding amendments and sections of the Act are also available. Find information about the cosmetic manufacturers list of public testing laboratories food labs role of FDA etc. The recent amendment in the Drug and Cosmetic Act shall will prevent pharmaceutical companies from marketing drugs manufactured by another pharmaceutical company by labeling their own company name hence preventing drug duplication.
Objective The DC Act was passed in 1940 10th April 1940 with the main object to Import Manufacture Distribution Sale of drug cosmetics. By Act 71 of 1986 sec2 wef. THE DRUGS AND COSMETICS ACT AND RULES THE DRUGS AND COSMETICS ACT 1940 as amended by the Drugs Amendment Act 1955 the Drugs Amendment Act 1960.
The Schedule J of the Drugs and Cosmetics Rules 1945 of India contains a list of diseases and ailments which a drug may not claim to prevent or cure.
Cosmetic Act 1940 Authorstream

Pharmacy Law Ethics And Regulatory Agencies Ppt Download

India Pharmaceutical Legal Regulatory Environment Food And Drug Law Institute Fdli
The Food And Drug Administration And The Federal Food Drug And Cosmetic Act Mercatus Center

Enforcement Of The Food Drug And Cosmetic Act Select Legal Issues Everycrsreport Com

Important Forms And Rules Of Drug And Cosmetic Act For Clinical Research Download Scientific Diagram

Legal Considerations Of Drugs Objectives Drug Standards Pharmacopoeia Food Drug And Cosmetic Act Ppt Download

Pure Imagination America S Food Identity Standards 1938 Present Uchri

Buy Federal Food Drug And Cosmetic Act The United States Federal Fd C Act Concise Reference Book Online At Low Prices In India Federal Food Drug And Cosmetic Act The United States

Part Ii 1938 Food Drug Cosmetic Act Fda

Summary Of New Drug New Fdc And Snd As Per Drug And Cosmetic Act Cliniexperts Cliniexperts
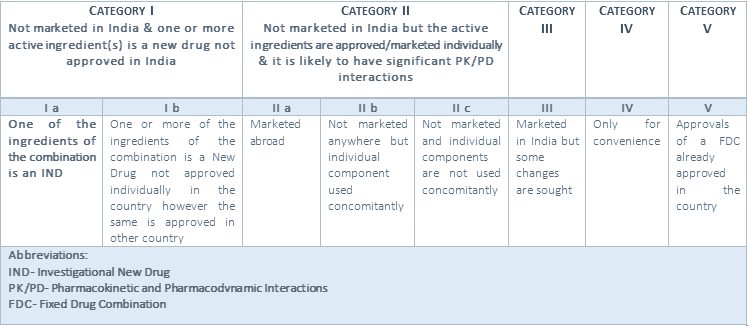
Summary Of New Drug New Fdc And Snd As Per Drug And Cosmetic Act Cliniexperts Cliniexperts

Labeling Guidelines For Cosmetics As Per The Drugs Cosmetics Act Nkg Advisory Business Consulting Services Pvt Ltd

Legal Considerations Of Drugs Objectives Drug Standards Pharmacopoeia Food Drug And Cosmetic Act Ppt Download

Approved Drug Products With Therapeutic Equivalence 39th Edition 2019 U S Government Bookstore

Buy Federal Food Drug And Cosmetic Act The United States Federal Fd C Act Concise Reference Book Online At Low Prices In India Federal Food Drug And Cosmetic Act The United States

U S Food Regulation Tales From A Twilight Zone The New York Times
Part Ii 1938 Food Drug Cosmetic Act Fda



Post a Comment for "Food Drug And Cosmetic Act India"