Food Drug And Cosmetic Act 505(j)
Bureau of Drugs a Federal Food Drug and Cosmetic Act PL. This letter is in reference to your abbreviated new drug application ANDA received for review on July 26 2016 submitted pursuant to section 505j of the Federal Food Drug and Cosmetic Act FDC Act for Icosapent Ethyl Capsules 1 gram.

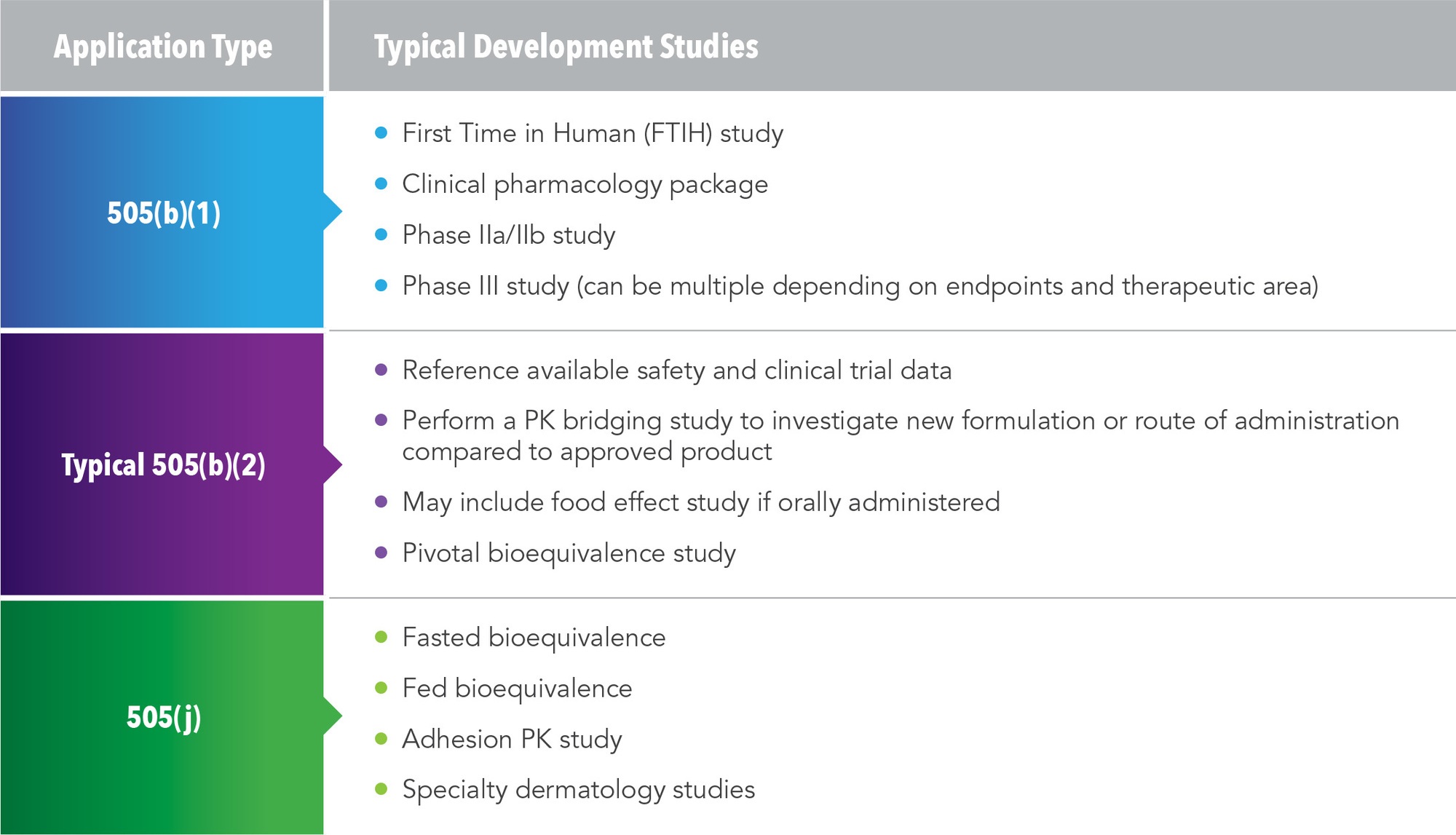
Approval Pathways Terminology And Requirements Download Table
75-717505 1938 and amended 21 Usc 355 Implied d Licenses for the maintenance ofestablishments for the propagationor manufacture and preparation of products described in subsectiona of thissection may b e issued only upon a showing that the establishment and the.
Food drug and cosmetic act 505(j). B The drug is limited by an effective application under section 505 of the Federal Food Drug and Cosmetic Act 52 Stat. Section 505j10 permits the Food and Drug Administration FDA to approve an abbreviated new drug application ANDA even if the ANDA approval coincides with. A 505j application available to an applicant who develops a modification of a drug.
301 as amended to use under professional supervision by a licensed health professional authorized to prescribe drugs unless it is dispensed only. Sultation with the Commissioner of Food and Drugs and experts in pediatric research shall develop prioritize and publish an annual list of approved drugs for which Ai there is an approved application under section 505j of the Federal Food Drug and. Provided That the provisions of section 701 section 371 of this title shall become effective on the enactment of this Act and.
A 505b2 application is a. Senior Director Drug Regulatory Affairs and Medical Affairs Dear Sir. Subchapter ivfood 341 350l1 subchapter vdrugs and devices 351 360fff8 subchapter vicosmetics 361 364 subchapter viigeneral authority 371 379dd2 subchapter viiiimports and exports 381 384g subchapter ixtobacco products 387.
321 For the purposes of this Act2. FEDERAL FOOD DRUG AND COSMETIC ACT As Amended Through PL. 301 This Act may be cited as the Fed-eral Food Drug and Cosmetic Act.
-- If the Secretary determines that the acceptance or approval of an application under section 505b2 or 505j for a new drug may occur after submission of reports of pediatric studies under this section which were submitted prior to the expiration of the patent including any patent extension or the applicable period under clauses ii through iv of section 505c3D or. The 45-day period provided for in section 505 c 3 C and j 5 B iii of the Federal Food Drug and Cosmetic Act does not apply. 9 The NDA is submitted as a 505b2 application for a drug that is a duplicate of a listed drug and is eligible for approval under section 505j of.
One section must contain the information described under paragraphs a2 through 6 and 8 and 9 of this section and section 505j2Avii of the Federal Food Drug and Cosmetic Act and. This guidance identifies the types of applications that are covered by section 505b2 of the Federal Food Drug and Cosmetic Act the Act. I Upon a written or electronic prescription.
1040 1938 21 USCA. 113233 Enacted December 16 2014 CHAPTER ISHORT TITLE SECTION 1. The Federal Food and Drugs Act of June 30 1906 as amended USC 1934 ed title 21 secs.
This codifies the existing regulations which require NDA holders to promptly notify FDA for removal of an Orange Book-listed patent for example if there has been a judicial finding of invalidity for a listed patent from which no appeal has been or can be taken. Section 31454 permits a 505b2 applicant to rely on the Agencys finding of safety. 115 shall remain in force until such effective date and except as otherwise provided in this subsection is hereby repealed effective upon such date.
The Federal Food Drug and Cosmetic Act and subsequent amending statutes are codified into Title 21 Chapter 9 of the United States Code. 2 If a drug product that contains a new chemical entity was approved after September 24 1984 in an NDA submitted under section 505b of the Federal Food Drug and Cosmetic Act no person may. Code 355 - New drugs.
52 rows Part A - Drugs and Devices sections 351 - 360n-1 FDC Act Section Number. No person shall introduce or deliver for introduction into interstate commerce any new drug unless an approval of an application filed pursuant to subsection b or j is effective with respect to such drug. Ii A statement as to whether according to the information published in the list the reference listed drug is entitled to a period of marketing exclusivity under section 505j5F of the Federal Food Drug and Cosmetic Act.
See Amendment to Section 505j7 of the Federal Food Drug and Cosmetic Act 21 USC. The Food and Drug Administration FDA or Agency is announcing the availability of a final guidance for industry entitled Citizen Petitions and Petitions for Stay of Action Subject to Section 505 q of the Federal Food Drug and Cosmetic Act. Among other things this guidance provides FDAs current thinking on what constitutes a 505 q petition and describes.

Referencing A Listed Drug For The 505 B 2 Pathway

Federal Register Abbreviated New Drug Applications And 505 B 2 Applications

Federal Food Drug And Cosmetic Act

How To Choose Between 505 B 1 505 B 2 Pathways
Part Ii 1938 Food Drug Cosmetic Act Fda

Two Year Countdown Begins For Fda Roll Over Of Biologics Currently Regulated As Drugs

Postmarketing Studies And Clinical Trials Implementation Of Section 505 O 3 Of The Federal Food Drug And Cosmetic Act Hartmannwillner

Fda C Gmp Training Program C Gmp In

Part Ii 1938 Food Drug Cosmetic Act Fda
White Paper Incorporating Innovation Into The 505 B 2 Development Pathway Evidera


Post a Comment for "Food Drug And Cosmetic Act 505(j)"